wonder heart liposomal coq10
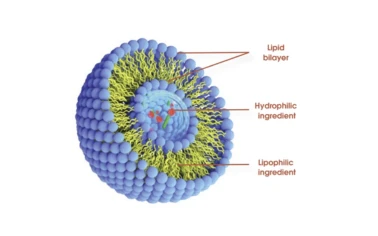
CoQ10 raw material: what savvy formulators are choosing in 2025 If you work in heart-health supplements or premium skin care, you already know the mitochondria talk isn’t hype. It’s manufacturing reality. The moment I sampled Coenzyme Q10 CoQ10 Powder Raw Material Cardiovascular Health Antioxidant Skin Care from Finutra, a few details stood out: consistent color, tight particle distribution, and—surprisingly—clean paperwork. Simple things, but they matter when your batch clock is ticking. At-a-glance specifications (real-world use may vary) Product Coenzyme Q10 (Ubiquinone), CAS 303-98-0 Assay (HPLC) ≥ 98.0% (USP 41) Particle size D90 ≤ 20 μm (≈ for standard grade); micronized options available Status Non-irradiated, ETO-free, NON-GMO Residual solvents Meets ICH Q3C by GC-MS Heavy metals Pb/Cd/Hg/As < 10 ppm total (ICP-MS) Shelf life 24–36 months sealed, N2-flushed drums, <25°C, dry, dark Origin Building 23B1, No.2 Yuanboyuan St., Zhengding Area of China (Hebei) Pilot Free Trade Zone Process flow (what’s behind consistent lots) Materials: ubiquinone produced via controlled fermentation and purification. Methods: multi-step crystallization → controlled drying → optional micronization for better dissolution. Testing: HPLC assay (USP 41), ID by UV/IR, GC-MS for solvents, ICP-MS for metals, PSD by laser diffraction, microbial counts per USP /. Packaging: light/oxygen barriers, nitrogen flush, tamper-evident seals. Industries: nutraceuticals, functional beverages, cosmetics (serums/creams), pharma adjuncts, pet wellness. Application scenarios - Cardiovascular: 100–200 mg capsules/softgels; many customers say dissolution consistency is the make-or-break. Finutra’s micronized grade helps. - Beauty: oil-phase serums and creams at 0.1–0.3% for antioxidant support; actually, pairing with vitamin E often boosts stability. - Beverages/gummies: consider cyclodextrin complexes or liposomal dispersions for bioavailability (customization on request). Vendor comparison (quick reality check) Vendor Certs / QA Lead time ≈ Customization Finutra (this product) USP 41 test std; ISO-based QA; Non-GMO; non-irradiated; ETO-free 7–15 days Micronized PSD, beadlets, complexes Trading House A Varies by lot; limited PSD data 15–30 days Minimal Lab-Only Supplier B Great docs; small batch 30+ days Niche forms; higher cost Case study: from R&D to market launch A mid-size EU supplement brand needed a cardiometabolic capsule with fast dissolution. We trialed micronized 98% ubiquinone; HPLC assay came in at 99.1%, D90 ≈ 12 μm. Stability at 40°C/75% RH over 6 months kept degradation under 3% with ascorbyl palmitate—solid for global shipping. Post-launch, they reported fewer batch deviations and, to be honest, happier QA meetings. In parallel, a skin-care client used 0.2% CoQ10 in an oil-in-water serum; in-use testing suggested improved skin radiance after 8 weeks (n=42, internal panel). Why this matters now Demand is surging in heart-health stacks and “inside-out beauty.” Coenzyme Q10 CoQ10 Powder Raw Material Cardiovascular Health Antioxidant Skin Care fits both: energy metabolism for the myocardium and antioxidant defense for photo-exposed skin. Plus, customers keep asking for ETO-free, non-irradiated inputs—that’s table stakes. Compliance and documentation Test Standard: USP 41 (HPLC assay, ID) Microbiology: per USP <61>/<62> Meets ICH Q3C for solvents; CoA + MSDS + Non-GMO + Allergen statements Halal/Kosher and ISO/GMP documents available on request If you need custom beadlets, lipid carriers, or cyclodextrin complexes, ask for pilot samples. Many customers say it speeds sensory and stability screening. And yes, Coenzyme Q10 CoQ10 Powder Raw Material Cardiovascular Health Antioxidant Skin Care ships nitrogen-flushed by default. References Mortensen SA et al. Coenzyme Q10 in chronic heart failure (Q-SYMBIO). JACC Heart Fail. 2014. doi:10.1016/j.jchf.2014.06.008 Zmitek K et al. Topical CoQ10 for skin—efficacy and penetration. Skin Pharmacol Physiol. 2017. doi:10.1159/000477425 United States Pharmacopeia. Coenzyme Q10 Monograph, USP 41. USP.org
Finutra devotes to be an integrated supplier for global supply chain, we offer a
broad array of raw materials and functional ingredients
Authoritative Certification
Continuous Innovation, Customer First
Enhance core competitiveness to bring customers better products and services,
Each of these is the result of our team's relentless pursuit of excellence
and our deep commitment to social responsibility.








Global
Reach
FINUTRA has over 350,000 square feet of manufacturing and warehouse
space worldwide.
Industries We Serve
Advanced molecular distillation and microencapsulation
technology. Extremely bioavailable
trace carotenoids Intuitively soluble.


STAY UPDATED
Receive special offers and first look at new
products.
products.
Building 23B1, No.2 Yuanboyuan St., Zhengding Area of China (Hebei) Pilot Free Trade Zone
QUICK LINK
Finutra devotes to be an integrated supplier for global supply chain, we offer a broad array of raw
materials and functional ingredients as a manufacturer, distributor and supplier for global Beverage,
Nutraceutical, Food, Feed and Cosmeceutical.
Copyright © 2025 Hebei Finutra
Biotech Co.,
Ltd. All
Rights Reserved.
Privacy Policy